How is dehydration different from distillation as a method of removing a solute from a solvent?
1 Answer
Dec 11, 2016
Refer to the explanation.
Explanation:
Dehydration involves the evaporation of the solvent from the solution, leaving behind a solid solute. It may be necessary to place the evaporating dish into a low-temperature oven to speed up the evaporation process.
Distillation involves differences in boiling point. The solute (a liquid) has a lower boiling point than the solvent. Once the solution starts boiling, the solute becomes a vapor, and then is passed through a condenser with cold water surrounding it, causing the vapor to condense back into the liquid solute (distillate) without the solvent.
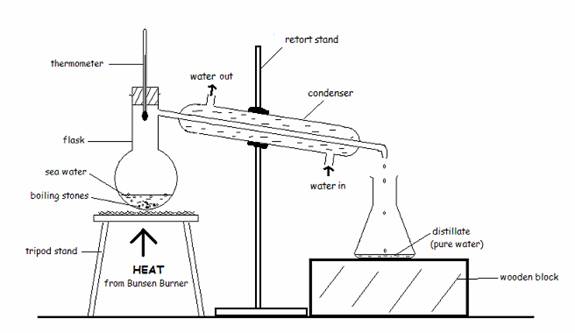
So dehydration removes the solvent and leaves behind the solute, and distillation removes the solute and leaves behind the solvent.

