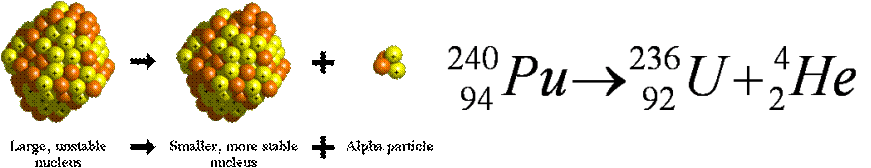How is the atomic mass changed by alpha decay?
1 Answer
May 18, 2015
Alpha decay involves the emission of an alpha particle by a radioactive isotope of an element. An alpha particle is a helium-4 nucleus,