How is the atomic number of an element determined?
1 Answer
Aug 19, 2017
By the number of protons found in one atom of an element.
Explanation:
The atomic number tells us the number of proton atoms in a single atom of an element. It is unique to each element.
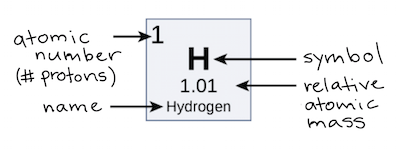
Do not be confused with isotopes, where an has the same number of protons (as its other isotopes), but a different number of neutrons.
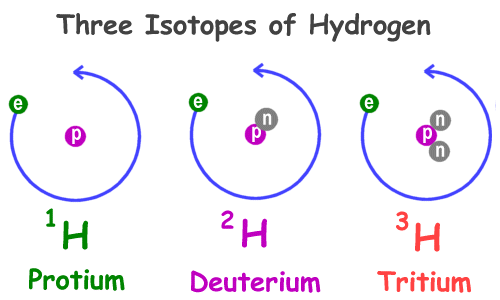
In this example, Hydrogen's atomic number remains the same - at 1. The only difference are the number of neutrons.
Hope this helps :)

