How is the Lewis structure of an ion written?
1 Answer
Jul 31, 2015
The same way you draw the structure of the original molecule or atom, except with
i.e. if charge is
So if you use the "counting valence electrons" method, you can draw
Total:
Count the electrons in here:
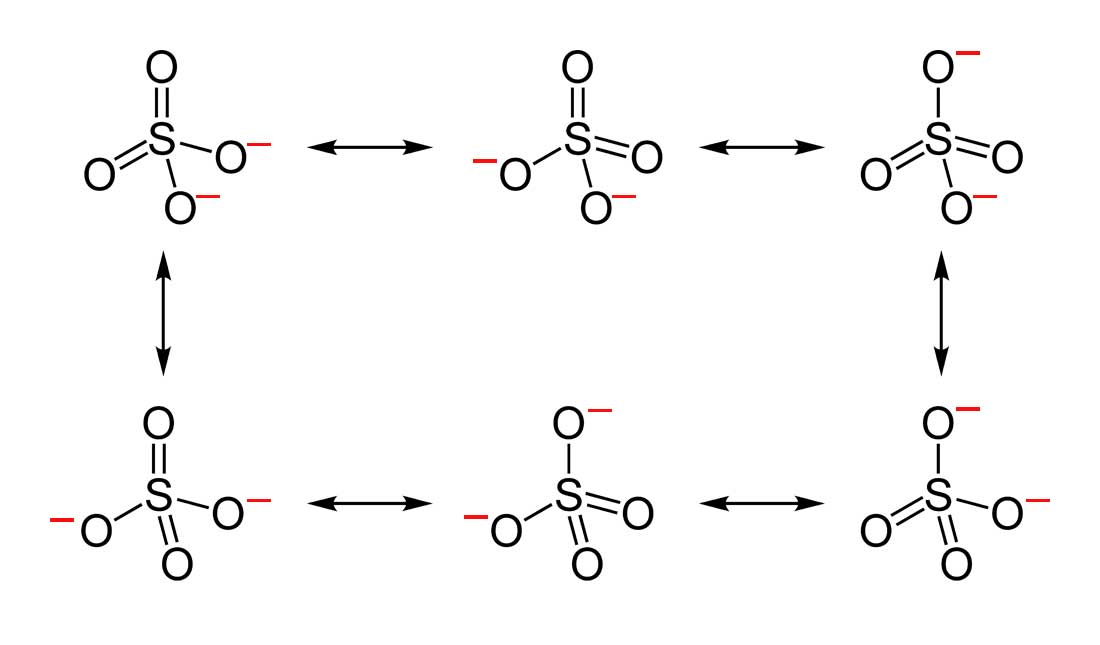
where the above is all of the resonance structures of
With formal charge defined here as
"Owned" electrons:
#6# per thionyl oxygen (#S=O# ), so#12# total.#7# per oxygen with a formal charge of#-1# (from#6 - 7# ), so#14# total.#6# per sulfur, so#6# total.
Sure enough, it matches:

