How many antibonding orbitals are there in Benzene?
1 Answer
Mar 24, 2014
There are three antibonding orbitals in benzene.
The six atomic p orbitals combine to form six molecular
The three lowest orbitals
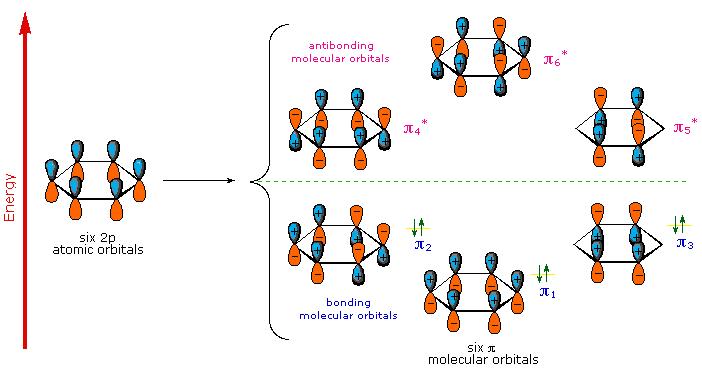
The remaining orbitals

