Why are antibonding orbitals filled first?
1 Answer
Jul 20, 2014
They aren’t — they are filled last.
An antibonding orbital is always higher in energy than its bonding counterpart.
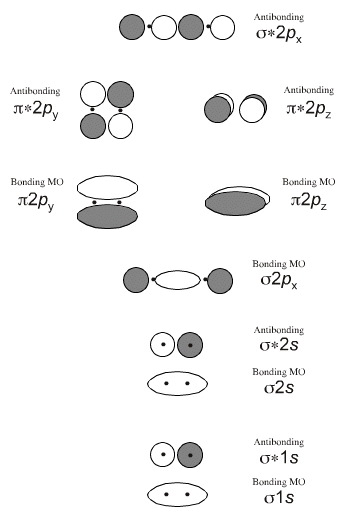
Thus, in terms of energy, σ1s <σ1s, σ2s <σ2s, σ2p < σ2p, and π2p < π2p.
But σ*1s < σ2s, for example. In this case, an antibonding orbital is filled before a bonding orbital.

