Bonding and Antibonding Orbitals
Key Questions
-
A bonding orbital is formed when two atoms approach each other and the overlap between atomic orbitals results in constructive interference.
The constructive interference causes the electron density between the two atoms to increase. This lowers the kinetic and potential energy of 1 or 2 electrons in the resulting bonding orbital. Because the energy of the system is lower when the atoms are near each other than when they are pulled apart, we say that a bond is formed.
-
Early atomic theories used the idea that an atom's electron(s) followed the same rules as a mini solar system where the planets were electrons orbiting a center proton sun.
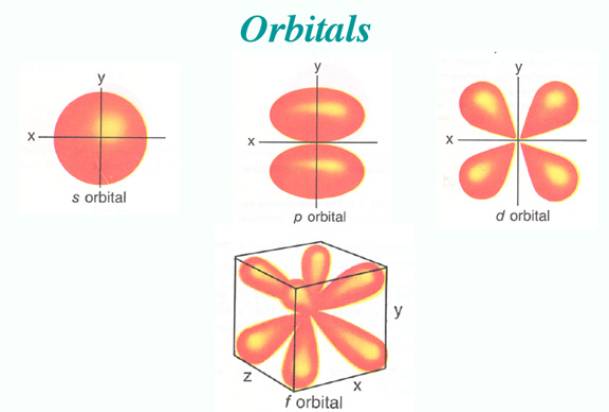
Early observations showed the electrons were moving more like a cloud surrounding the nucleus rather than an individual planet. They also have a wobbly shape. The shape of the cloud, or orbital, depended on the amount of energy, angular momentum and magnetic moment of the individual electron.
The properties of an atom's electron configuration are described by four quantum numbers: n, ℓ, m, and s.
The rules the electrons follow to orient themselves around their atom are simple once the rules governing the quantum numbers are understood..
There are 4 types of orbitals.