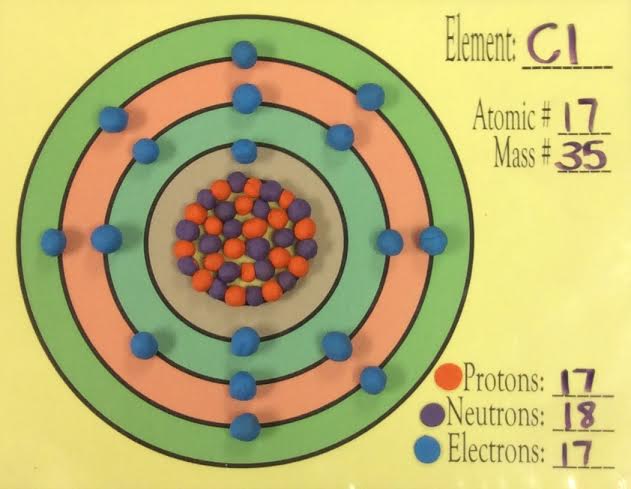
How many electrons go on each ring according to the Bohr model?
1 Answer
Electrons in the Bohr model follow the number of elements in the rows of the periodic table.
2 in the first orbit
8 in the second and third ring
18 in the fourth etc...
Explanation:

The Bohr model is a simplistic means of explains the placement of the subatomic particles of the elements of the periodic table.
The Bohr model arrangement of electrons follows the same pattern as the periodic table.
2 elements in the first row, 2 electrons in the first orbit.
8 elements in the second row, 8 electrons in the second orbit.
8 elements in the third row, 8 electrons in the third orbit.
The fourth and fifth orbits would therefore hold 18 electrons.
For most chemists the Bohr model does not work well beyond the third row of the periodic table because the model become quite oversized and bulky.