You will need to refer to a solubility curve graph, which indicates how much of each solute will dissolve in #100"g"# water at indicated temperatures. A typical one looks like this:
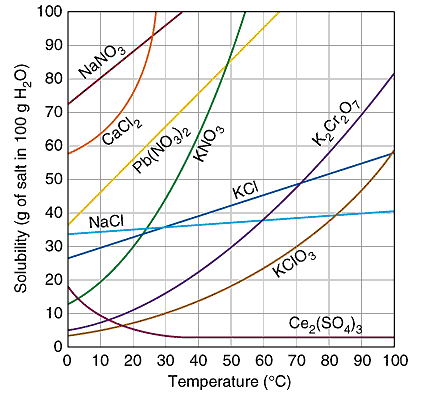
We need to look at how much #"CaCl"_2# will dissolve in #100"g" "H"_2"O"# at #10^oC#.
From the graph, the point of intersection between the #"CaCl"_2# curve (the orange line) and the #10^oC# line appears to occur at the point of roughly #64"g"# per #100"g"H_2O#
This means that at #10^oC#, #64"gCaCl"_2# will dissolve in #color(black)(100"gH"_2"O"#, but the question asks how much will dissolve in #color(black)(200"gH"_2"O"#. This can be found simply enough by doubling the solubility at #10^oC#:
#64"gCaCl"_2 xx 2 = 128"gCaCl"_2#.
So, #128"gCaCl"_2# will dissolve in #200"gH"_2"O"# at #10^oC#

