How many half-lives will it take to reach 6.25% of its original concentration?
If A(g) #-># B(g) has k=#2.3*10^-3# #s^-1# , how many half-lives will it take for the concentration of A to reach 6.25% of its original concentration?
If A(g)
2 Answers
Mar 28, 2018
It will take 4 half lives.
Explanation:
1st gets you to 50%
2nd gets you to 25%
3rd gets you to 12.5%
4th gets you to 6.25%
Mar 28, 2018
Explanation:
#"A"_"(g)" -> "B"_"(g)" color(white)(...)"k" = 2.3 × 10^-3\ "s"^-1#
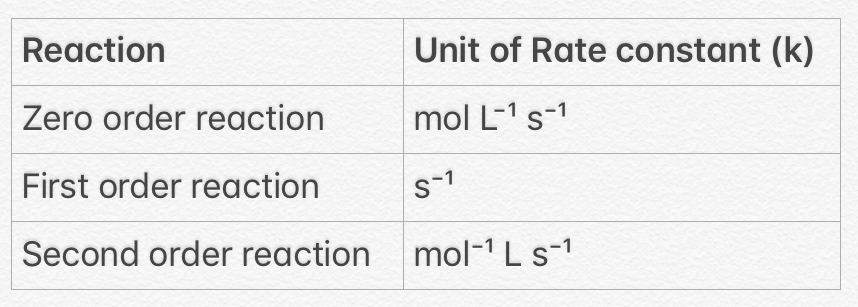
Here, unit of Rate constant
Units of Rate constant in zero, first and second order reactions.
For a first order reaction, we can write
#"N" = "N"_0/2^"n"# Where
#"N"_0 =# Initial amount of substance#"N ="# Amount of substance after#"n"# half lives#"n ="# Number of half lives
∴ It takes

