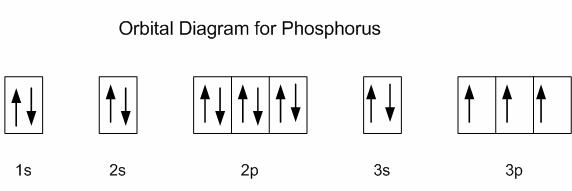
How many orbitals are in the 4p subshell?
1 Answer
Oct 31, 2015
three (
Explanation:
It does not matter if your energy level (that is, the coefficient/number before the spdf orbital) goes as high as 7 (which is, by far, the maximum), the number of suborbitals in
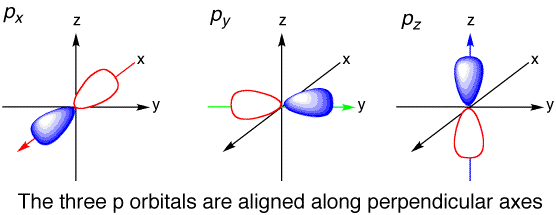
Take a look at the illustration below. The number of orbitals for p did not change regardless if its