How many pi bonds are in #SO_2#?
1 Answer
There are two
Explanation:
First, let us consider the structure of the
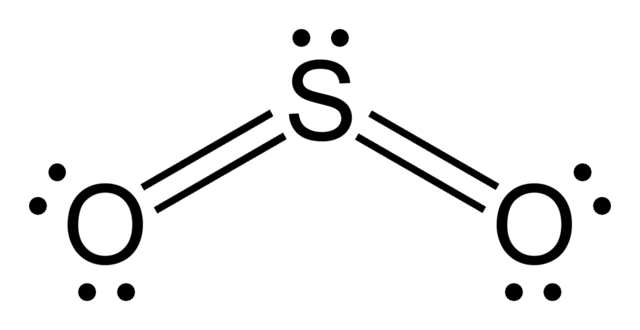
As you can see, the molecule is bent / v-shaped / angular, and there are three regions of electron density: two
Now, recall that the composition of a double bond is as follows:
- 1x
#sigma# bond - 1x
#pi# bond
Therefore, with two double bonds we have two
The
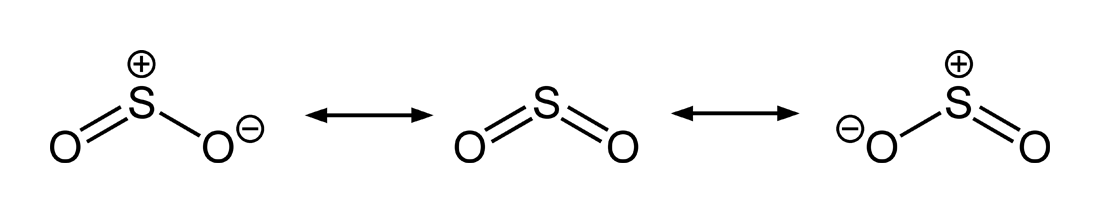
In molecular orbital theory, this is more commonly thought of as a delocalised

