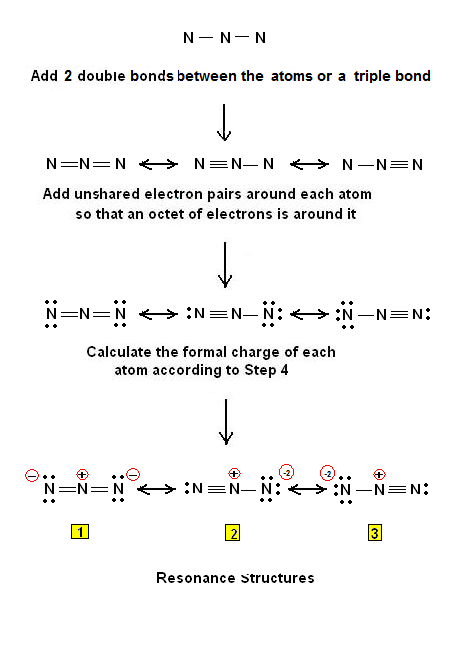
How many resonance structures does N3- have?
1 Answer
3 resonance structures
Explanation:
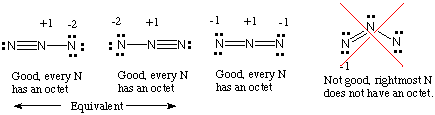
You must first know how many valence electrons are in one
Now that we know the number of valence electrons per element, it is just a matter of drawing the electron dot configuration. The total number of electrons involved is calculated by adding the valence electrons involved (multiplied by the subscript) plus the ion's overall charge.
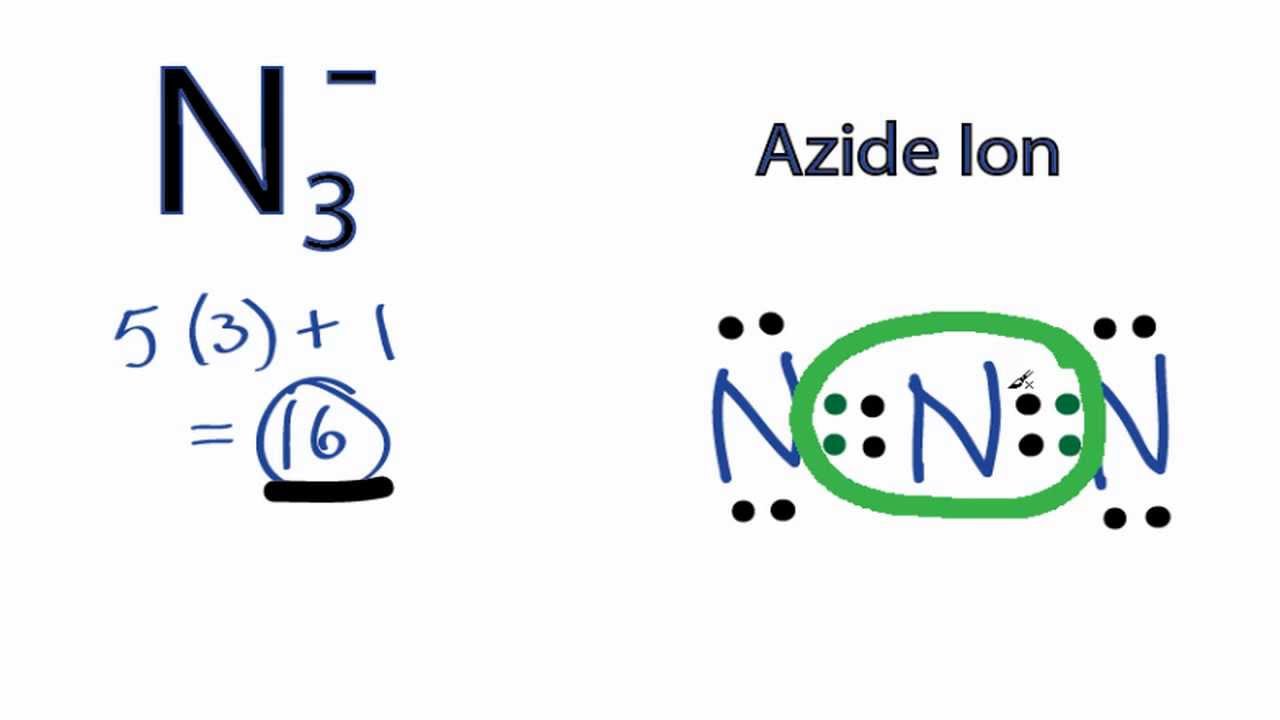
Always remember that resonance structures also must follow the octet rule (eight electrons per atom).