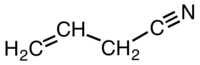
How many sigma and pi bonds are in allylcyanide?
1 Answer
Jul 6, 2016
I got:
#\mathbf(9)# #sigma# bonds#\mathbf(3)# #pi# bonds
"Allyl" means there's a
So, allyl cyanide is:
Or, drawn out in full:
Each single bond contains only one sigma (
#\mathbf(3)# #"C"-"C"# #sigma# bonds#\mathbf(1)# #"C"-"N"# #sigma# bond#2+1+2 = \mathbf(5)# #"C"-"H"# #sigma# bonds
Now counting the
Therefore, we have:
#\mathbf(1)# #"C"-"C"# #pi# bond#\mathbf(2)# #"C"-"N"# #pi# bonds
So, we have a total of:
#\mathbf(9)# #sigma# bonds#\mathbf(3)# #pi# bonds