How many sigma and pi bonds are in benzene?
1 Answer
I count:
bb6 xx (sp^2) "C"-"C" sigma (sigma ) bondsbb6 xx (sp^2) "C"-"H" sigma (sigma ) bondsbb3 xx (sp^2) "C"="C" pi (pi ) bonds
Benzene is an aromatic compound, one of whose major resonance structures is depicted like so:
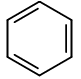
The other major resonance structure is the horizontal reflection over the vertical axis, so the overall resonance hybrid structure, which represents benzene most accurately in real life, is more like this:
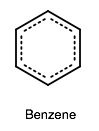
One way we can count each
(all implicit hydrogens are shown. There are no other implicit hydrogens in the full structure.)
From this, recall that one single bond contains one sigma bond. The sigma (
6 xx (sp^2) "C"-"C" sigma (sigma ) bonds6 xx (sp^2) "C"-"H" sigma (sigma ) bonds
Then, when we incorporate the additional electrons that are delocalized throughout the ring, it is easiest to count the pi (
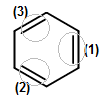
A pure double bond, if you recall, contains one sigma (
We've accounted for all the
bb6 xx (sp^2) "C"-"C" sigma (sigma ) bondsbb6 xx (sp^2) "C"-"H" sigma (sigma ) bondsbb3 xx (sp^2) "C"="C" pi (pi ) bonds

