How many sigma and pi bonds are in #C_4H_6#?
1 Answer
Apr 16, 2016
Nine sigma and two pi bonds are present.
Explanation:
There are a number of isomers of
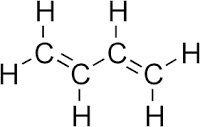
1,3-butadiene has four carbon atoms, joined together with two double bonds and a single bond. A double bond is a
There are six hydrogen, each with only a single bond joining them to the carbons, and so this means another
Putting all of this together,

