The structural formula and IUPAC name of the given compound is
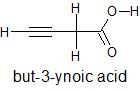
To know the no. of #sigma and pi" bonds" # the Lewis structure of the compound is to be drawn showing all single,double and triple bonds. In this structure
#" Each single bond is 1"sigma" bond"#
#" Each double bond is 1"sigma and 1 pi" bond"#
#" Each triple bond is 1"sigma and 2 pi" bond"#
Using this information we can get the no. of #sigma and pi" bonds" # in the given structure
#7" single bonds"->7sigma " bonds"#
#1" double bond"->1sigma and 1pi " bonds"#
#1" triple bond"->1sigma and 2pi " bonds"#
#color(red)("So we have total"->9sigma and 3pi " bonds here")#


