How many sigma and pi bonds are present in a molecule of cumulene?
1 Answer
Apr 9, 2016
Explanation:
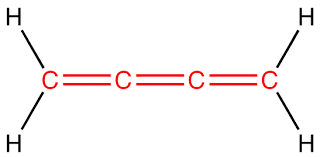
This is a molecule of cumulene,
Single bonds are one
Cumulene has three double bonds and four single bonds, or,

