How many sigma and pi bonds do sp, sp2, sp3, sp3d, sp3d2 have?
1 Answer
Without knowing more context, it's not possible to know the number of
If you want to get the number of
One pure double bond has one
From knowing the hybridization of the central atom, we can determine the number of
Examples:
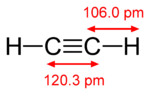
#sp# hybridization in acetylene corresponds with two#sigma# bonds around one carbon.
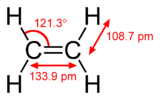
#sp^2# hybridization in ethene corresponds with three#sigma# bonds around one carbon.
#sp^3# hybridization in methane corresponds with four#sigma# bonds around one carbon.
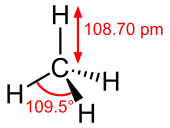
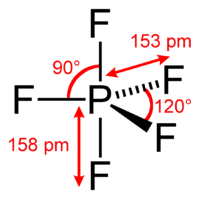
#sp^3d# hybridization in#"PF"_5# corresponds with five#sigma# bonds around one phosphorus.
#sp^3d^2# hybridization in#"SF"_6# corresponds with six#sigma# bonds around one sulfur.
You can see that the number of orbitals used in the hybridization spits out the same number of hybridized orbitals that can
Therefore, the number of orbitals used in the hybridization is the number of

