How many sigma (σ) bonds and pi (π) bonds are in formaldehyde, #H_2CO#?
1 Answer
Apr 22, 2016
Explanation:
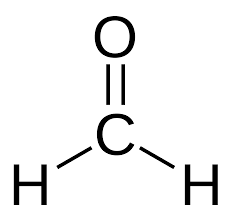
A
Here, for example, there are
A double bond is equal to
Putting this all together,

