How many sigma bonds are in benzene?
1 Answer
May 22, 2016
Benzene is a six-membered aromatic ring.
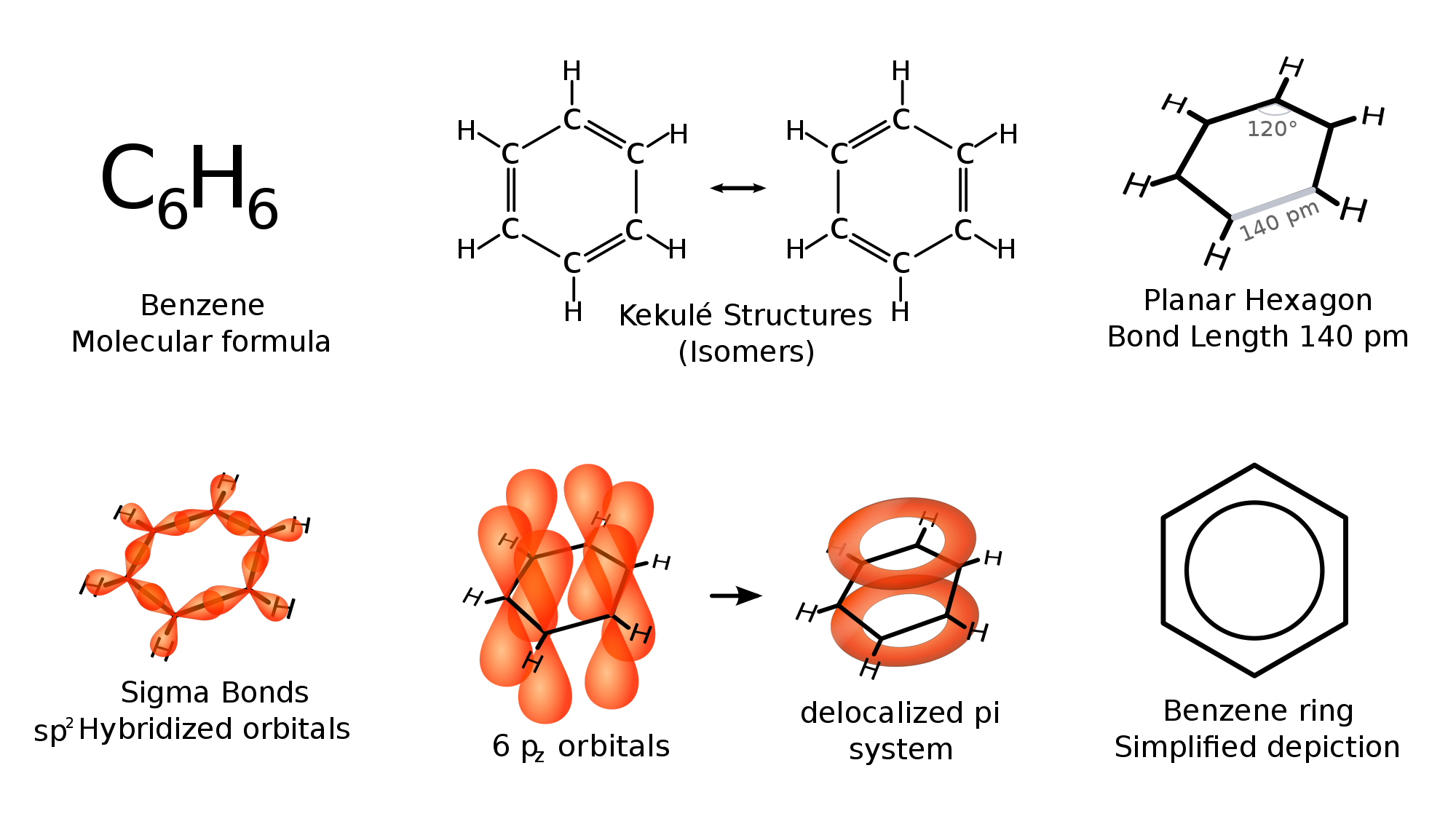 https://upload.wikimedia.org/
https://upload.wikimedia.org/
Any sigma (
Every double bond or single bond will contain exactly one
Thus, regardless of the depiction of benzene, whether it's the conjugated, delocalized, or "metal nut" depictions, it contains 6

