How many sp2-hybridized carbons are in the structure of norethindrone?
1 Answer
May 31, 2016
There are three
Explanation:
The structure if norethindrone is
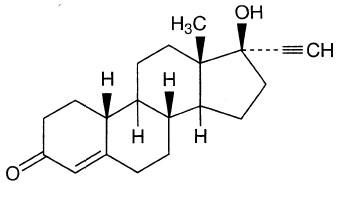
(from dailymed.nlm.nih.gov)
Every carbon that is part of a double bond system is
We see that there are only three such carbon atoms, and they are all in the lower left hand ring.
Two of them are the atoms in the
The third is the

