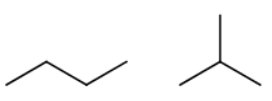The formula for butane is #"C"_4"H"_10#.
Thus, the formula #"C"_4"H"_10"O"# tells us that the compound is saturated, so it can be either an alcohol or an ether.
Isomeric alcohols
First, draw all the isomers of butane.
You get
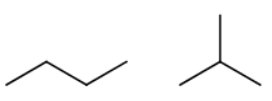
#color(white)(mm)"butane"color(white)(mmm)"and"color(white)(mm)"isobutane"#
Second, put an #"OH"# group on every carbon atom that does not give you a duplicate structure.
You get

#color(white)(mm)"butan-1-ol" color(white)(mmm)"butan-2-ol" color(white)(m)"2-methylpropan-1-ol"color(white)(m)"2-methylpropan-2-ol"#
Ethers
Draw the isomers of butane as before and put an #"O"# atom between every possible carbon that does not give a duplicate structure.
You get
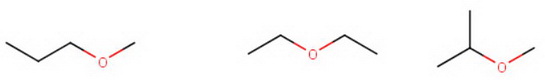
#"methyl propyl ether"color(white)(mmmmmmm)"diethyl ether"color(white)(mm)"isopropyl methyl ether"#