How do you solve a stoichiometry problem?
1 Answer
You use a series of conversion factors to get from the units of the given substance to the units of the wanted substance.
Explanation:
There are four steps in solving a stoichiometry problem:
- Write the balanced chemical equation.
- Convert the units of the given substance (A) to moles.
- Use the mole ratio to calculate the moles of wanted substance (B).
- Convert moles of the wanted substance to the desired units.
The flow chart below summarizes the process.
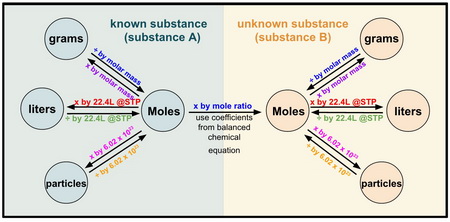
(From MillingsChem)
NOTE: The mole ratio of A to B is central to all the calculations.
EXAMPLE:
What mass of chlorine does the decomposition of 64.0 g of AuCl₃ produce?
Solution:
1. Write the balanced chemical equation.
2. Convert grams of
3. Use the molar ratio to convert moles of
4. Convert moles of

