How would the molecular (microscopic) level of salt and sugar look when dissolved in water?
1 Answer
Mar 21, 2017
Well,
Explanation:
When an ionic salt dissolves in water (or some other polar solvent), bond breaking is conceived to occur. For salt, in water, the strong electrostatic bond between
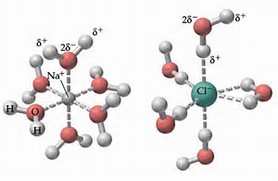
On the other hand, when sugar dissolves in water, NO chemical bonds are broken, and the polar hydroxyl groups of the sugar molecule are solvated by water molecules.
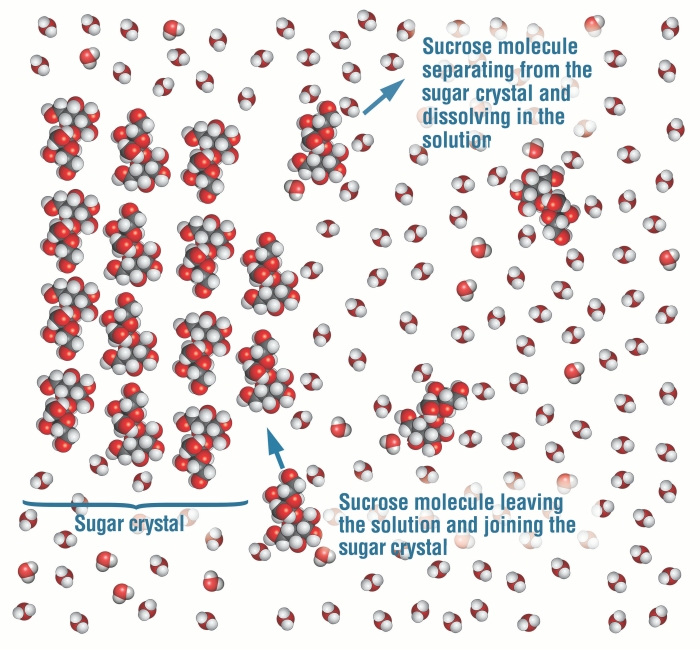
Please note that the pictures are CONCEPTIONS; they are not a physical structural representation, such as an X-ray structure would provide.

