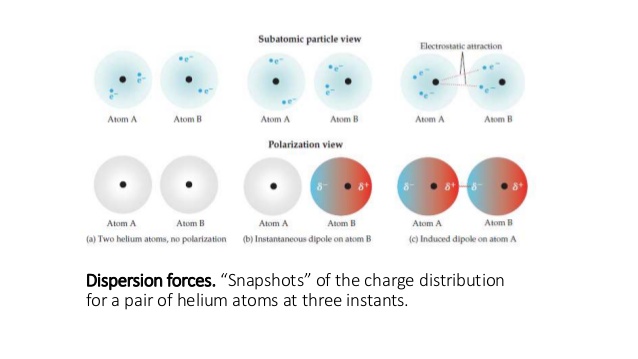
How would you define dispersion force?
1 Answer
May 25, 2017
See below.
Explanation:
London dispersion forces are considered the weakest intermolecular type of Van der Waals forces.
They are present in an instantaneous electric dipole moment in non-polar as well as polar molecules.
This type of force becomes stronger as the atom enlarges, and to a smaller degree for large molecules. This is due to the increased polarizability of molecules with more dispersed electron clouds where free electrons can be found.