How would you differentiate between monosubstituted and disubstituted alkenes?
1 Answer
I would use infrared spectroscopy to differentiate the alkenes.
Explanation:
The infrared
However, alkenes have characteristic, strong out-of-plane bending vibrations in the region below
Monosubstituted alkenes give two strong peaks in this region; disubstituted alkenes give only one.
Compare these values with the spectra below.
Monosubstituted
Hex-1-ene (monosubstituted) has strong peaks at
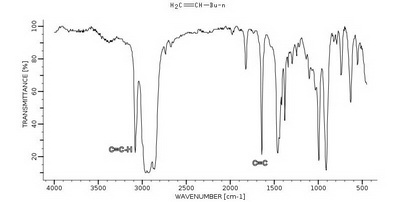 Hex-1-ene
Hex-1-ene
(from chemistry.umeche.maine.edu)
cis-Disubstituted
cis-Oct-2-ene has a strong peak at
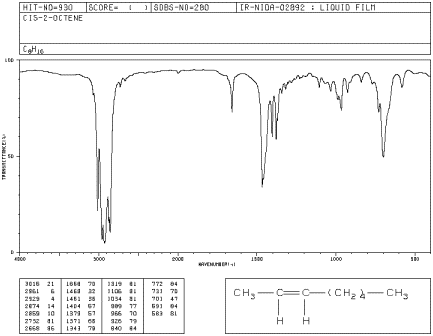 employees.csbsju.edu
employees.csbsju.edu
trans-Disubstituted
trans-Oct-2-ene has a strong peak at
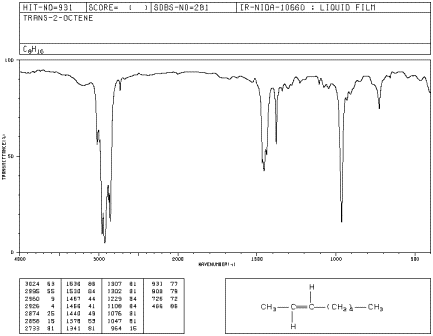 employees.csbsju.edu
employees.csbsju.edu
gem-Disubstituted
2-Methylpent-1-ene has a strong absorption has a strong absorption at
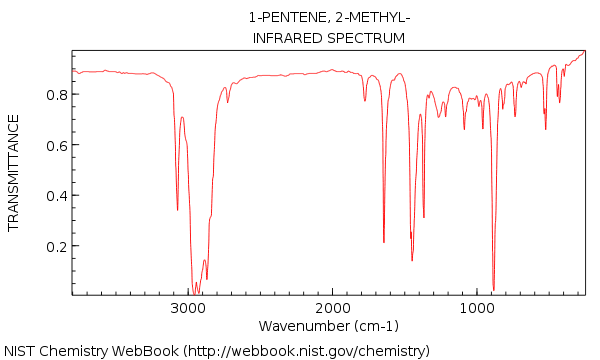 2MePent1
2MePent1
(from webbook.nist.gov)

