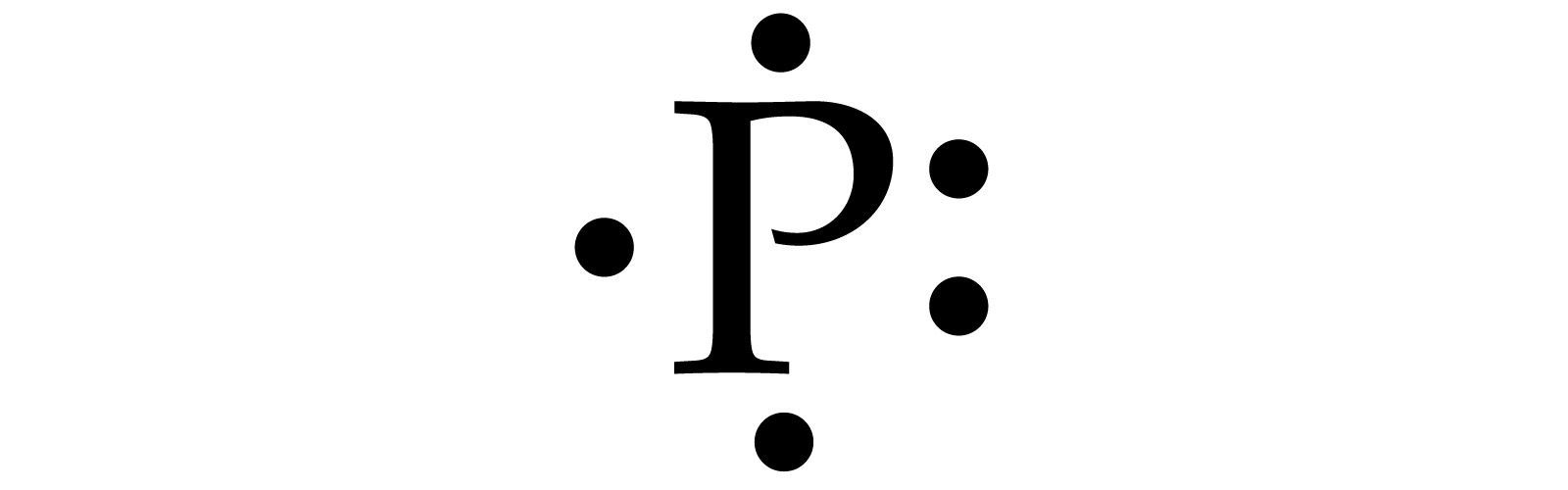How would you draw a Lewis structure for an atom that has the electron configuration #1s^2 2s^2 2p^6 3s^2 3p^3#?
1 Answer
Feb 4, 2018
A P with 5 dots surrounding it.
Explanation:
Based on your electron configuration, the element that you want to draw is Phosphorus, since you have 15 electrons. Phosphorus has 5 valence electrons.
To draw Lewis Structures for elements, the symbol for the element is drawn with the number of valence electrons it has surrounding it. So, to draw the Lewis Structure, begin by drawing the symbol for Phosphorus, the letter P. Next, Phosphorus has 5 valence electrons. So start with one dot on top, then one dot to the right, one dot on the bottom, one dot to the left, and another dot on top, next to the first one.
I learned to draw them like above, but your drawing could also look something like this.