How would you draw all the resonance structures for nitrate, NO3-?
1 Answer
Explanation:
First is you need to know the number of valence electrons. You can do this by (1) drawing the electron configuration per element or (2) consulting your periodic table.
If you chose to draw the electron configuration per element, you will have something like this:
If you chose to consult your periodic table, just notice that
Now that you know the number of valence electrons per element, you need to compute the total valence electrons for the
5 + (3 x 6) = 23 electrons
But since the whole molecule has a -1 charge, you need to add this too. So the total number of valence electrons is 24.
The next thing to do is draw. Normally, the first element in the chemical formula is the central atom. In this case, the
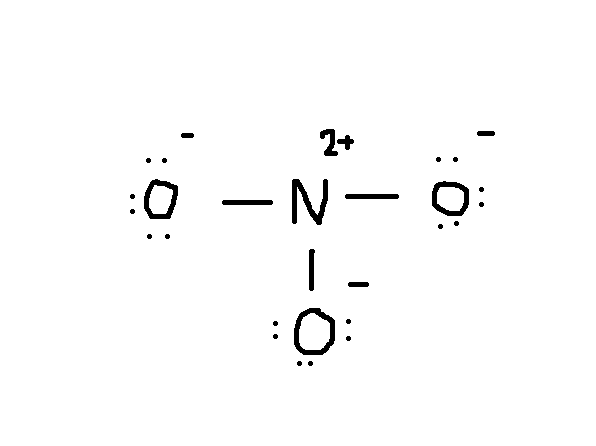
Notice that the lone pair of electrons from
Add another bond, but where?
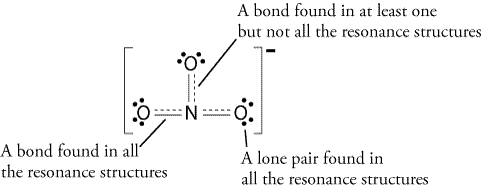
Therefore, the resonance structure would look like this:
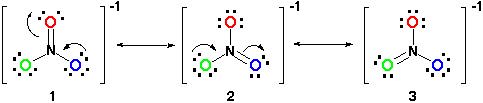
All the resonance structures are correct since it all follows the octet rule and all have a total number of 24 electrons. Just take note that the only bond moving is the pi (

