How would you explain the difference between van der Waals interactions and hydrophobic interactions?
1 Answer
The hydrophobic force arises from the disruption of hydrogen bonds between water molecules, whilst van der Waals interactions are the result of instantaneous and induced dipoles .
Explanation:
The Hydrophobic Force
-
Let us consider the shape of a water molecule: it is a bent molecule consisting of two
Furthermore, the high electronegativity of oxygen means that the
If a non-hydrogen bonding surface is introduced, such as oil, this network is disrupted. As such, the water molecules redistribute themselves so that as few hydrogen bonds as possible are disrupted. This typically occurs more and more readily as the entropy,
Van der Waals Interactions
Van der Waals interactions arise from the temporary dipoles that occur due to random movement of electrons in the orbitals - the regions of space they may occupy - within an atom or molecule. The changing distribution of the electron cloud leads to certain areas of the species being slightly positive in charge (
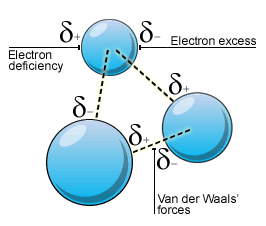
This instantaneous dipole distorts the electron clouds of local complexes, resulting in induced dipoles withing them. These induced dipoles then induce temporary dipoles in other local complexes. Of course, being temporary, this dipole instantly ceases to be as the movement of electrons causes the electron cloud to be redistributed; however, this results in a new instantaneous dipole and so the induction of new induced dipoles.

