How would you predict the ideal bond angle(s) around each central atom in this molecule?
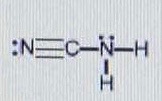
1 Answer
Jul 11, 2016
You look at the number of electron groups, and consider how to distribute these groups evenly in space.
CENTRAL CARBON
- The carbon has two electron groups, so a
#180^@# bond angle is the furthest even separation between the two atoms.
CENTRAL NITROGEN
- The right nitrogen is the interesting one; with four electron groups (the lone pair, the carbon, and the two hydrogens total four), it has a tetrahedral electron geometry and a trigonal pyramidal molecular geometry in accordance with VSEPR Theory.
These geometries have ideal bond angles of
#109.5^@# in three dimensions, but the lone pair 'crunches' the atoms together a little, so the angles become less than#109.5^@# .This molecule is similar to
#"NH"_3# with one hydrogen replaced with#"C"-="N"# . Since#"C"-="N"# is larger than#"H"# , the#"C"-"N"-"H"# bond angle should be slightly larger than the#"H"-"N"-"H"# bond angle of#"NH"_3# , but less than#109.5^@# .

