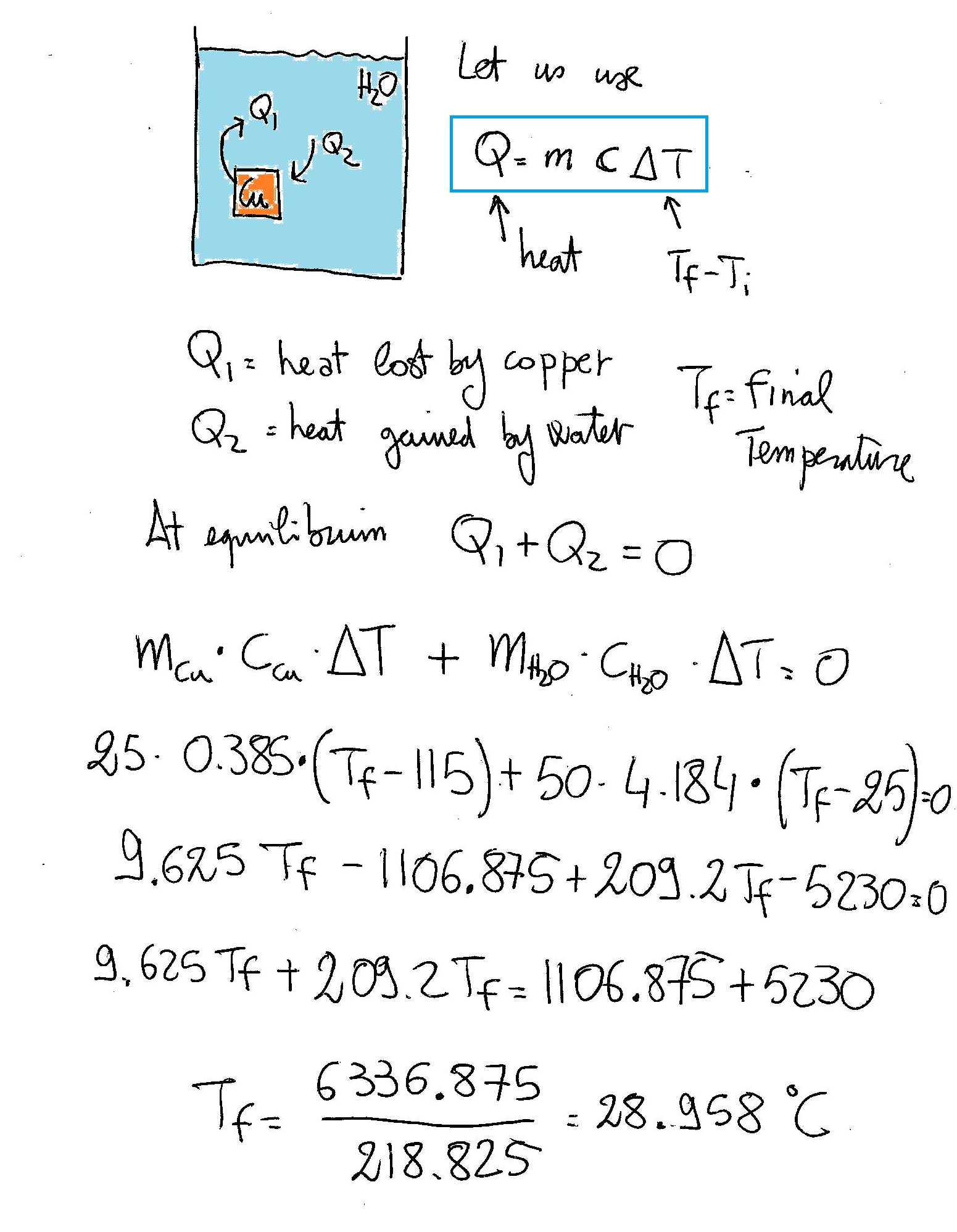
If a #25.00g# piece of copper (#C_s = (0.385J)/(g*°C)#) at #115.0°C# is added to #50.00g# of water (#C_s = (4.184J)/(g*°C)#) at #25.00°C#, what is the final temperature once the object and water thermally equilibrate?
1 Answer
Jul 14, 2017
I got almost
Explanation:
Have a look: