If reaction #A-> B# is exothermic, how does the activation energy for the forward reaction compare with the activation energy for the reverse reaction, #B -> A#?
1 Answer
In the case of an exothermic reaction, the activation energy of the forward reaction will always be smaller than the activation energy of the reverse reaction.
In your case, the forward reaction will look like this
Since heat is released during the reaction, the product will have a lower energy than the reactant. You go from a higher energy level, as was the cse for
The difference in energy between
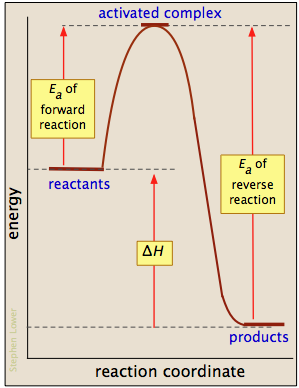
In the case of the reverse reaction, you're going to have
You're going to have to supply energy to the more stable
The difference between the activation energy of the forward reaction and the activation energy of the reverse reaction will be
You need to supply to
This means that you have
For exothermic reactions, you'll always have a bigger

