Iodine-131 is a radioactive isotope with a half-life of 8 days. How many grams of a 64 g sample of iodine-131 will remain at the end of 24 days?
2 Answers
Explanation:
The nuclear half-life of a radioactive isotope tells you how much time must pass in order for half of the atoms present in an initial sample to undergo radioactive decay.
In essence, the half-life tells you at what time intervals you can expect an initial sample of a radioactive isotope to be halved.
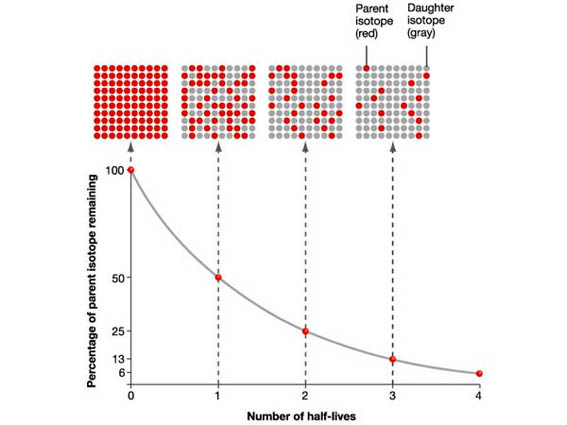
In your case, iodine-131 is said to have a half-life of
If you take
#1/2 * A_0 = A_0/2 -> # after the passing of one half-life
#1/2 * A_0/2 = A_0/4 -># after the passing of two half-lives
#1/2 * A_0/4 = A_0/8 -># after the passing of three half-lives
#vdots#
and so on.
You can thus say that the amount of iodine-131 that remains undecayed,
#color(blue)(|bar(ul(color(white)(a/a)A = A_0 * 1/2^ncolor(white)(a/a)|)))#
Here
In your case, you have
#n = (24 color(red)(cancel(color(black)("days"))))/(8color(red)(cancel(color(black)("days")))) = 3#
This will get you
#A_"24 days" = "64 g" * 1/2^3#
#A_"24 days" = color(green)(|bar(ul(color(white)(a/a)"8 g"color(white)(a/a)|)))#
Explanation:
Half Life follows first Order kinetics
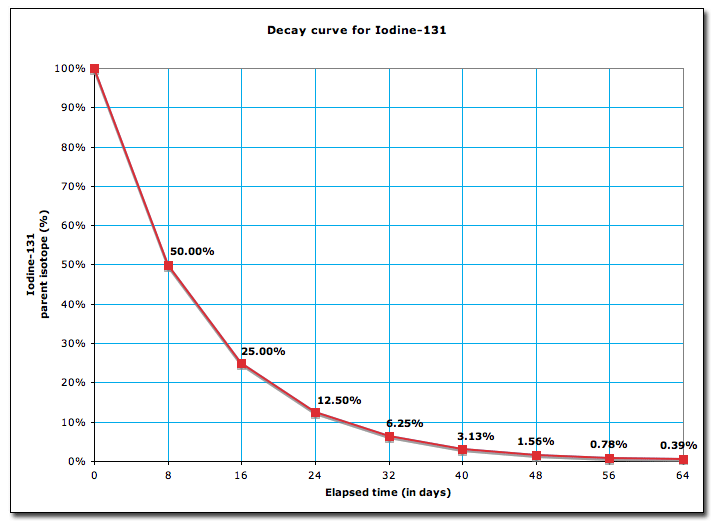
This graph shows what is going on during the decay
As you can see from the graph the half life is 8 days
Lets do the math and verify it with the graph
Recall
With this you can solve most problems regarding the above data
Now
Greater the concentration of a sample greater the mass in in it
So
Verifying with the graph
Hence verified


