Is CuCl_2CuCl2 an ionic salt or covalent molecule?
2 Answers
Explanation:
Chlorine has a high electro negativity of 3.0. Copper like most metals has a low electro negativity, So the bonding is ionic making the compound an ionic salt.
Explanation:
Here is my reasoning.
A. Electronegativity differences
The electronegativity of
This is much less than the normal cutoff value of 1.7 for declaring a compound ionic.
It predicts only about 33 % ionic character — i.e. a polar covalent
B. Crystal structure
The crystal consists of
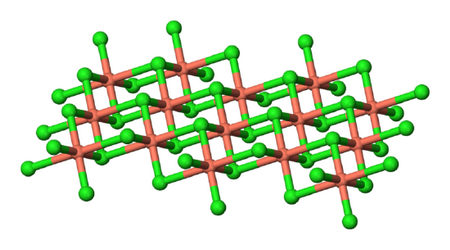 CuCl2
CuCl2
(From Chemistry LibreTexts)
C. Colour
 Comparison
Comparison
(From Amazing Rust.com)
In contrast,

