Linkage isomers nitropentaamminecobalt(iii)chloride shows 2 bands at 1065 and 1470 nitritopentaamminecobalt(iii)chloride shows 2 bands at 1300 and 1430 on infrared spectrum. How do you account for these differences?
1 Answer
Feb 6, 2016
Nitro is coordinated by the
Explanation:
I think you may have reversed the IR assignments.
The structures of the two isomers are
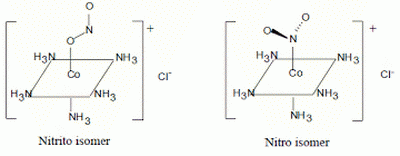
(from www.vgloop.com)
The two
Their stretching vibrations are coupled to give
The asymmetric stretch is very strong, and the symmetric stretch is weak.
The nitrito isomer has an
Both peaks are very strong and well separated, with

