Of single, double, and triple covalent bonds, which is the longest? Which is the strongest?
1 Answer
What do you think...?
Explanation:
The modern chemical bond is conceived to be a region of high electron density between two positively charged atomic nuclei which NEGATES electrostatic repulsion between the positive charges, and a net attractive force operates that binds the nuclei together.
In a single bond, electron density is situated BETWEEN the nuclei...in a double bond the electron density lies in PLANES above and below the atom-atom vector...and for a triple bond the electron density lies normal to the plane of the double bond... (and yes there are quadruple bonds, but I am not going to consider it here)…
This is best addressed pictorially...
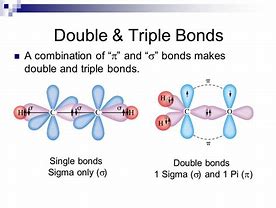
And typical carbon-carbon lengths...
And the greater the bond order, the greater the bond strength, and the SHORTER the bond....
I will leave you to find the values associated with

