On the periodic table, why are atoms in group 1 likely to form ions with a +1 charge?
1 Answer
Because they have one electron in their outermost shell.
Explanation:
The electron configuration of an atom, which as you know is influenced by that atom's location in the periodic table, determines what type of ion that atom can form.
More specifically, you know that chemical reactivity is driven by an atom's desire to obtain a stable electron configuration, that is, eight electrons (or two, in some cases) on its outermost shell - this is known as having a complete octet.
Group 1 metals, which are known as alkali metals, have one electron on their outermost shell, as you can see here
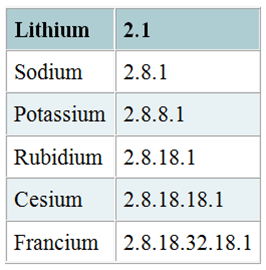
This means that it's much, much easier for a group 1 atom to lose that electron than to, say, gain seven more, in order to complete its octet.
Moreover, that solitary electron is located furthest from the atom's nucleus, and it's being screened by the nucleus' positive charge by the electrons located in the lower energy shells.
All these factors contribute to the atom's tendency to lose that outermost electron and form a

