If each orbital can hold a maximum of 3 electrons, the number of elements in the 4th #bb"[period of the]"# periodic table (long form) is?
In brackets is a fix to the question.
- Truong-Son
In brackets is a fix to the question.
- Truong-Son
2 Answers
Well, this is kind of open to interpretation... but if I interpreted it correctly, I get
(If you already knew that the number of electrons allowed in a given orbital is derived from the properties of electrons, and NOT of the orbitals themselves, the answer quickly follows.)
PRELIMINARY THINGS
I think there's a typo in the question... I looked this up elsewhere, and it's probably...
If each orbital can hold a maximum of 3 electrons, the number of elements in the 4th period of the periodic table (long form) is...?
Also, note that this is entirely theoretical, as all electrons only have two possible spins (
That aside, when we suppose three electrons are "allowed" in a single orbital (assuming the other three quantum numbers are as normal), we suddenly "allow"
(It's not really why the periodic table was arranged historically, but... I suppose that's what the intent of this question was...)
EXPANDING THE PERIOD...
An electron configuration for the fourth period in a generalized manner is written as:
#4s^x 3d^x 4p^x# where:
#x = (2l+1) cdot N_(m_s)# is the total number of electrons in all the orbitals in a given subshell.#l# is the angular momentum quantum number.#l = 0, 1, 2, 3, . . . # corresponds to#s, p, d, f, . . . # orbitals.#2l+1# is known as the degeneracy of the subshell; it is how many orbitals are in that subshell.#N_(m_s)# is an arbitrary number of spins the electron could have, as it also then gives the maximum number of electrons per orbital. In this case we SUPPOSE that#N_(m_s) = 3# , but in real life it is just#2# .
Now, the number of allowed
For the
#l = 0, => 2l+1 = 1#
For the
#l = 2, => 2l+1 = 5#
For the
#l = 1, => 2l+1 = 3#
Thus, the hypothetical electron configuration we would then write is...
#4s^(1cdot3) 3d^(5 cdot 3) 4p^(3 cdot 3)#
#= 4s^3 3d^15 4p^9#
And that would apparently expand the fourth period of the periodic table from
This is what I get.
Explanation:
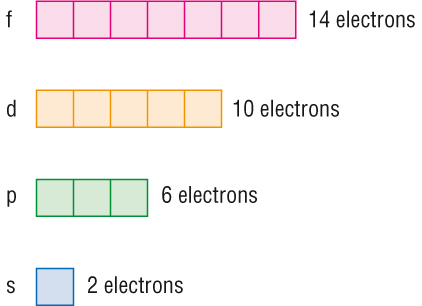
We see that on the assumption that each orbital holds maximum of
Total number of electrons held in
If each orbital is allowed to hold
Total number of electrons held in this shell
This is the number of elements allowed in
.-.-.-.-.-.-.-.-.-.-.-.-.-.-
Alternatively
Total number of electrons

