Snow and Ice are both solid forms of water,then why doesn't snow have such a solid structure as ice and why do their colors differ? Even sometimes we see snowy white color in some parts of an ice cube,but snow doesn't appear transparent from anywhere.why?
2 Answers
A) Snow and ice DO have the same solid structure, chemically. B) “Color” is our reception of light waves at different frequencies. It is affected by both molecular and macro-structures.
Explanation:
Solid water forms with a consistent structural framework. However, how the crystals “connect” depends on the environment. Liquid water forms a continuous block of solid as it freezes. Water in the air crystallizes into much smaller accumulations of ice (snow).
Thus, the spaces between water molecules and the macro-patterns (ice chunk, sleet, or snow flake) will take on different shapes.
Perceived color is a result of the diffraction and reflection of light through and off of a substance.
The random jumble of individual (or clumped) snow flakes will create a different light scattering pattern than a more structured block of solid ice.
Snow and Ice are both solid forms of water and, therefore, are chemically same. However, there are differences in the formation of each which results in the perceived difference in the physical appearance of snow and ice crystals.
Snow is precipitation of water in the form of crystalline flakes. As such snow is ice formed directly from water vapor in the earth's atmosphere. Figure below shows snow crystals of various variety of hexagonal sizes and shapes.
Snow is composed of small ice particles. As such it is a granular material. It has an open and therefore soft, white, and fluffy structure. This gives its physical appearance and characteristic color.
Ice on the other hand is a crystalline phase of water formed from its solidification from liquid state.
Ice too has predominantly hexagonal based crystal structures.
The domain of the crystalline structure of snow is limited to the snowflake whereas ice crystals have considerably larger domains often limited by size of the container. Figure below shows the hexagonal crystal lattice of ice. (The hexagonal form known as ice Ih is the only one that is found on the surface of the earth at normal temperatures and pressures.)
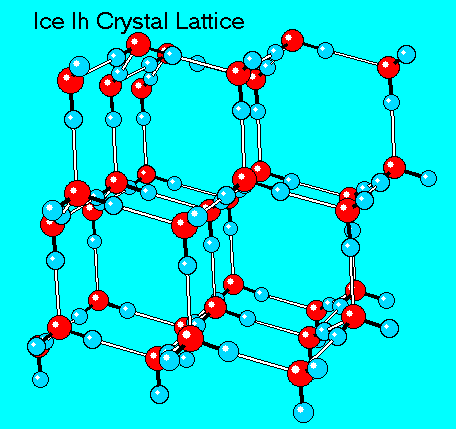
Depending on the presence of impurities ice can appear transparent or more or sometimes less opaque.
Figure above shows frozen water in the form of an ice cube. The white opaque zone in the center is result of tiny air bubbles.

