The carbon dioxide molecule contains how many double bonds?
1 Answer
Jun 16, 2018
Explanation:
We got carbon dioxide, with
Formally, the carbon centre is
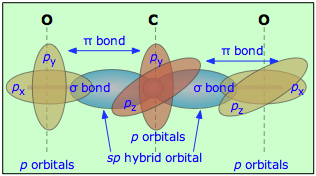
And clearly, the two
We got carbon dioxide, with
Formally, the carbon centre is

And clearly, the two