The density of titanium metal is 4.59 g/cm3 at 25 C. What mass of titanium displaces 204.03 mL of water st 25C?
1 Answer
The mass of titanium is
Explanation:
Density is the amount of mass per unit volume. The density equation is
If you know two of the variables you can calculate the third.
In this case you know density,
We can use the density and volume to calculate the mass of titanium.
Rearrange the equation to isolate mass. Substitute the given values into the equation and solve.
The following diagram is a density triangle. It is a tool to help you calculate density.
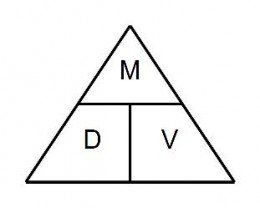
The way it works is to cover the missing variable with with your finger and observe the relationship between the other two variables.
For example, if you don't know the volume, put your finger over the

