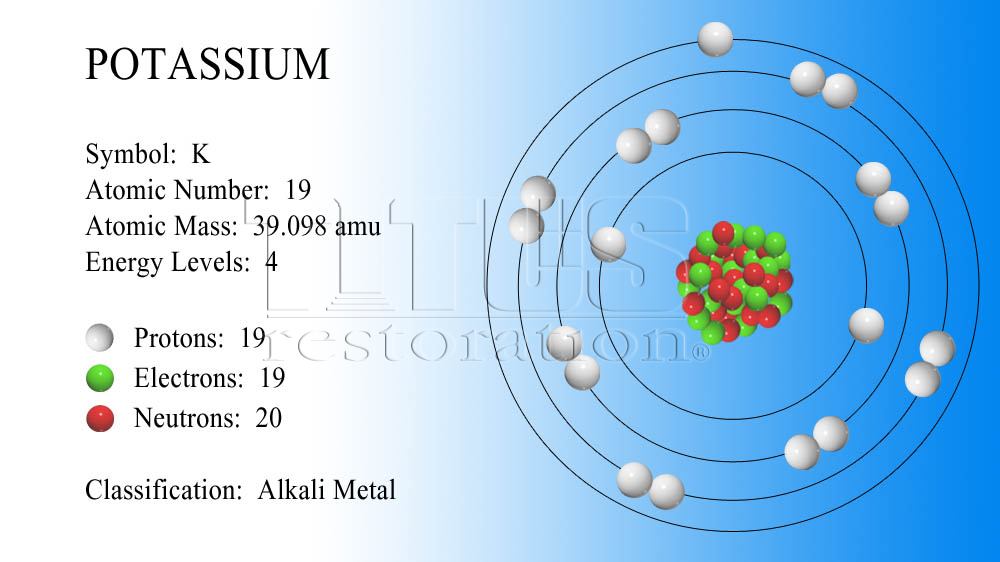
The element potassium's atomic number is 19. Its relative atomic mass is 39.098. How many protons occur in a potassium atom?
1 Answer
Explanation:
This is a perfect example of a problem that wants to test your understanding of a basic concept.
An element's atomic number tells you how many protons are present inside that element's nucleus. As you can see, the answer is given away in the question, provided that you know what the atomic number is all about.
You can thus say that an atom of potassium contains
You can use the relative atomic mass,
To do that, simply take the value of the relative atomic mass and round it to the nearest integer. This will get you the mass number of the most abundant potassium isotope.
In your case, you have
39.098 ~~ 39
This tells you that the most abundant isotope of potassium contains
39 - 19 = "20 neutrons"
 http://quoteaddicts.com/topic/potassium-quotes/
http://quoteaddicts.com/topic/potassium-quotes/
You now know that the most abundant isotope of potassium contains

