The four single bonds of a carbon atom in #CH_4# are directed toward the corners of what shape?
2 Answers
A tetrahedral.
Explanation:
Methane,
A two-dimensional drawing of methane:
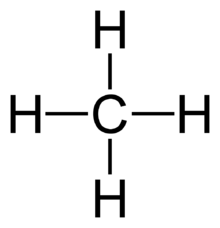
We see that methane has no lone pairs, as predicted (saturated hydrocarbon).
When we assign molecular geometry and electron pair arrangement, we consider the steric number of the central atom and how many lone pairs it possesses. The steric number is the number of atoms bonded to the central atom plus the number of lone pairs on the central atom. The central atom of methane is carbon, which has no lone pairs and a steric number of
Because this molecule has no lone pairs of electrons, its electron pair arrangement is the same as its molecular geometry. The molecular geometry of a molecule with a steric number of
A perspective drawing of methane:
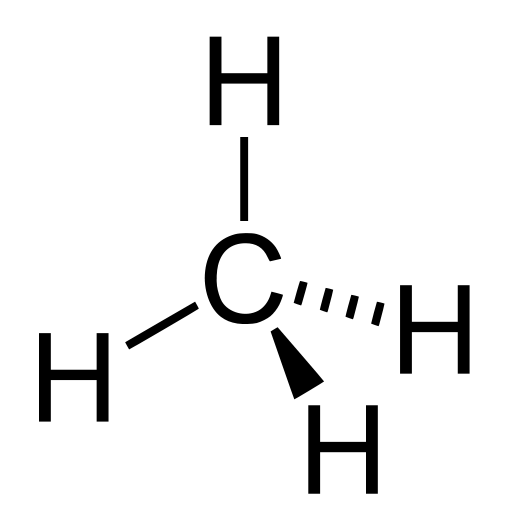
Explanation:
Eicosohedra and dodecahedra, 20 and 12 sided solids respectively, complete the 5 Platonic solids.
In a regular tetrahedron, such as described by the


