The gas inside of a container exerts #15 Pa# of pressure and is at a temperature of #450 ^o K#. If the temperature of the gas changes to #90 ^oK# with no change in the container's volume, what is the new pressure of the gas?
1 Answer
Jun 13, 2016
The new gas pressure is
Explanation:
For this type of question, you would use Gay-Lussac's Law
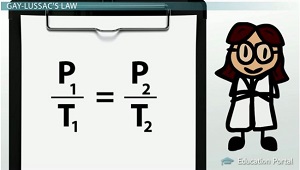
Gay-Lussac's Law shows that there is a direct relationship between pressure and temperature as long as the volume and number of moles of gas remain unchanged.
Let's start off with identifying our known and unknown variables.
The first pressure we have is
Now we just rearrange the equation in order to solve for
#P_2 = (T_2 * P_1)/(T_1)#
#P_2 = 3 Pa#
P.S. When using the Kelvin scale, you do not put the degree symbol. You just write K.

