The half-life of P-32 is 14.30 days. How many milligrams of a 20.00 mg sample of P-32 will remain after 85.80 days?
1 Answer
Aug 4, 2017
The amount remaining is
Explanation:
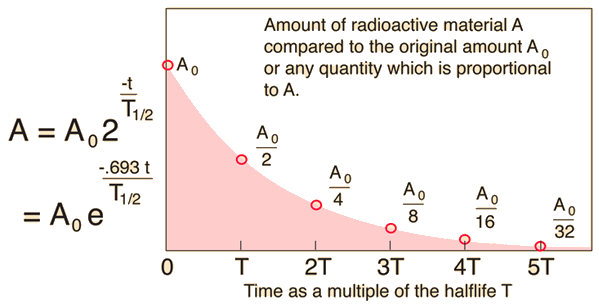
The half life is
This means that after
The time is
This is equal to
Therefore,
The amount remaining is
We can solve this problem with the equation
The radioactive constant is
Therefore,

