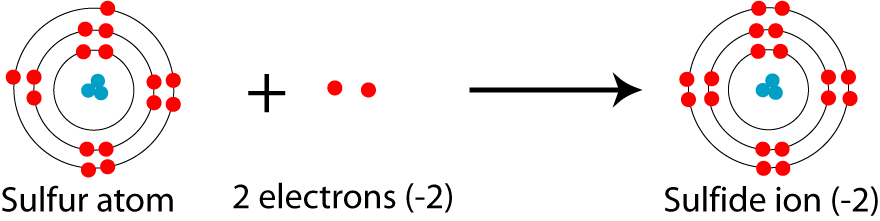
The net charge on a sulfide ion, #"S"^(2-)#, is #2-#. How does this ion obtain its charge?
1 Answer
A neutral sulfur atom gains
Explanation:
An ion can only be formed when a neutral atom gains or loses electrons.
Since electrons carry a negative charge, gaining electrons will result in the formation of a negatively charged ion, or anion. Similarly, losing electrons will result in the formation of a positively charged ion, or cation.
In your case, the sulfide anion,
More specifically, it gained
For each electron gained, the ion's overall charge decreases by