The rate of solvolysis of # CH_3COCl # is greater than # CH_3CH_2Cl#. why?
1 Answer
Here's my understanding of the reaction.
Explanation:
Solvolysis is the reaction of a substrate with the solvent.
For example, if the solvent is methanol, the solvolysis of acetyl chloride would be represented as:
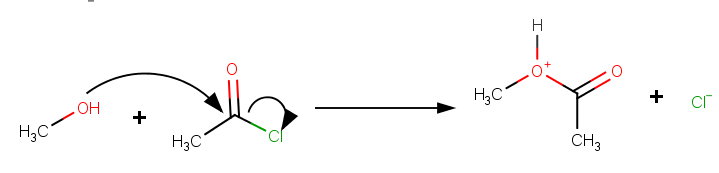
The mechanism involves an in-plane
It appears to be an
If we compare this reaction with the solvolysis of ethyl chloride (below), we see two differences.
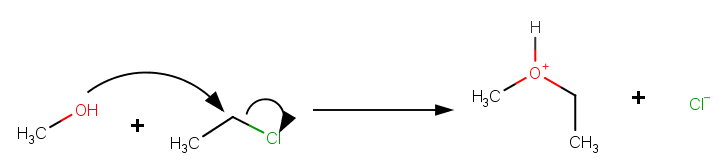
(1) The carbonyl carbon in acetyl chloride is
The carbonyl carbon has more
This weakens the
(2) The carbonyl carbon has a resonance contributor that puts more positive charge on the carbon atom.
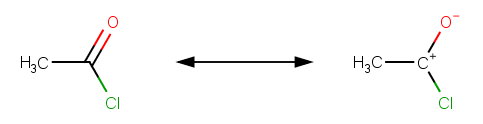
This makes the carbonyl more susceptible to attack by a nucleophile. It lowers the activation energy, so the reaction is faster.

