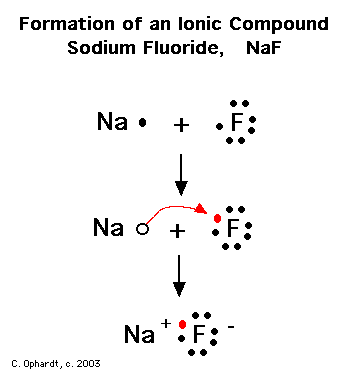
The strongest interactions in the compound sodium fluoride, NaF, are an example of what type of bond?
1 Answer
Dec 10, 2016
Sodium fluoride is an ionic compound, in which there is an ionic bond between oppositely charged ions.
Explanation:
During the formation of