What are elimination reactions?
1 Answer
In general, an elimination reaction (specifically, it's called
Note that they don't necessarily all happen in one step.
REACTION ORDER
We have a first-order and a second-order process associated with elimination. These are called
E1 REACTIONS vs. E2 REACTIONS
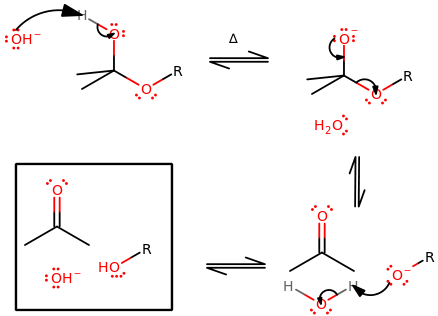
Some examples can be seen below.
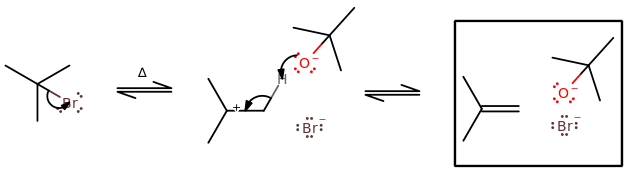
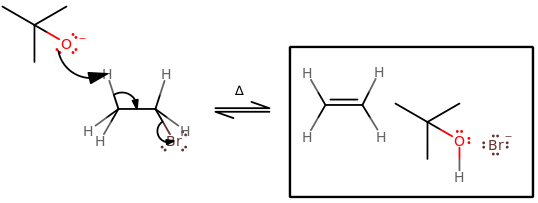
The key features of these two are:
- A proton from the
#beta# carbon leaves, forming a#pi# bond across a carbon-carbon bond such that the most substituted product is made (Zaitsev's Rule). - Favorable at higher temperatures.
- Favorable for higher steric hindrance on the electrophile and/or nucleophile.
The key differences are:
#"E"1# has no need for an antiperiplanar orientation, but#"E"2# does.#"E"1# has a carbocation intermediate, which allows for 1,2-hydride shifts or 1,2-alkyl shifts, but#"E"2# does not have or allow either.
WHEN YOU HAVE A POOR LEAVING GROUP
Lastly, under